A. Using Performance Objectives
This manual contains performance objectives for each of the 20 lab exercises covered in BIOL 230. The objectives tell exactly what you are expected to perform after the completion of each lab exercise.
When verbs such as "define," "state," "discuss," "describe," or "differentiate" are used in the objective, you will be expected to "perform" that objective by way of short answer, multiple choice, or matching questions on a pre-announced quiz. When verbs such as "demonstrate" or "perform" are used, you will be expected to demonstrate a particular technique or procedure to the instructor during the course of the laboratory exercise. When verbs such as "recognize" or "interpret" are used, you will be expected to give a written interpretation of the results of an experiment when given these results in either a written form or a practical form.
As a general rule, when the objective falls under the discussion sections of a lab exercise, it will be tested by means of short answer, multiple choice, or matching questions; when an objective falls under the procedure section of a lab exercise, it represents a procedure or technique that must be mastered during the course of the lab period; and when an objective is found under the results section of an exercise, it will most likely be tested for by a practical question.
Return to Menu for Introduction
B. Laboratory Rules
For the safety and convenience of everyone working in the laboratory, it is important that the following laboratory rules are observed at all times:
1. Anyone working with bacterial cultures, chemicals, and/or projectile and sharps hazards must wear:
a. Approved (ANSI Z87.1) safety goggles;
b. Gloves; and
c. A lab coat buttoned all the way to the bottom.Goggles and gloves will be provided in the lab but you must purchase your own lab coat at the college bookstore. Lab coats cannot be removed from a microbiology lab. They will be stored in the lab and must be discarded at the end of the semester.
2. Place only those materials needed for the day's laboratory exercise on the bench tops. Purses, coats, extra books, etc., should be placed in the lab bench storage areas or under the lab benches in order to avoid damage or contamination.
3. Since some of the microorganisms used in this class are pathogenic or potentially pathogenic (opportunistic), it is essential to always follow proper aseptic technique in handling and transferring all organisms. Aseptic technique will be learned in Laboratory 2.4. No smoking, eating, drinking, or any other hand to mouth activity while in the lab. If you need a short break, wash or sanitize your hands and leave the room.
5. If you should spill a culture, observe the following procedures:
a. Immediately place the culture tube in the plastic baskets found in the hood in the back of the room so no one else touches the contaminated tube.b. Have your partner place paper towels over the spill and spray liberally with Lysol. After 10 minutes, place the paper towels in the biohazard container.
c. Both you and your partner wash your hands with disinfectant soap and sanitize your hands.
d. Notify your instructor of the spill.
6. Report any cuts, burns, or other injuries to your instructor.7. Use only the provided pens or pencils in the lab. Any pen or pencil you use during the lab should be wiped down with Lysol before returning them.
8. Using a wax marker, properly label all inoculated culture tubes or Petri plates with the name or the initials of the microorganism you are growing, your initials or a group symbol, and any other pertinent information. It is important to know what microorganisms are growing in each tube or on each plate.
9. Place all inoculated material only on your assigned incubator shelf, the shelf corresponding to your lab section. Culture tubes should be stored upright in your dedicated test tube rack, while Petri plates should be stacked and incubated upside‑down (lid on the bottom) in the Petri plate holder.
9. After completing an experiment, dispose of all material properly:a. Place all culture tubes upright in the plastic baskets found in the disposal hood. Lay them in the basket carefully so they do not tip over and spill.
b. Place Petri plates in the plastic bag-lined buckets found in the disposal hood.
c. Put all used pipettes, swabs and microscope slides in the biohazard disposal containers located in the front of the room and under the hood.
10. Tie back long hair.
11. Do not touch the face, apply cosmetics, adjust contact lenses, or bite nails.
12. Handle all glassware carefully. Notify your instructor of any broken glassware (culture tubes, flasks, beakers, etc.) or microscope slides. DO NOT PICK UP BROKEN GLASSWARE WITH YOUR HANDS! Use the dust pan and brush. All broken glassware must be disposed of in the sharps/biowaste container in room D-202S.
13. Use caution around the Bunsen burners. In a crowded lab it is easy to lean over a burner and ignite your hair or clothing. Keep the burner well away from the edge of the lab bench to avoid hair or clothing from contacting the flame.
14. Always clean the oil from of the oil immersion lens of the microscope with a piece of lens paper at the completion of each microscopy lab.
15. You must wear shoes that cover the tops of your feet to prevent injury from broken glass, spilled chemicals, and dropped objects. Sandals are not permitted in the lab!
16. When doing any lab where microbes are being stained for viewing with a microscope, make sure the used dye in the staining tray is poured into the waste dye collection container, not down the sink.
17. Return all equipment, reagents, and other supplies to their proper places at the end of each lab period.
18. Disinfect the bench top withLysol before and after each lab period.
19. Always wash and/or sanitize your hands with soap and water for 20 seconds before leaving the laboratory.
20. Do not run in the laboratory. Avoid horseplay.
21. To avoid contamination and damage, do not use cell phones or other personal media devices in the laboratory.
22. Please read the laboratory exercises and follow the laboratory directions carefully.
23. Do not place regular trash such as Kim wipes and paper towels in the biohazard containers.
IN CASE OF EMERGENCY, CONTACT CAMPUS PUBLIC SAFETY AT (443) 840- 1111 and describe the situation and your location.
Students who engage in any actions that may damage college property, create an unsafe condition, injure another person, or result in a disruption that interferes with learning may have any, or a combination of the following sanctions imposed as determined by the instructor:
a. A verbal or written warning;
b. Being directed to leave the class for the remainder of the period;
c. A referral to either the Campus Ombudsman or the Department Chairperson;
d. Suspension from the class or the college.
Please see Code of Conduct in the most recent Student Handbook.
Return to Menu for Introduction
C. Personal Protection Equipment (PPE)
The following personal protection equipment must be worn in all microbiology labs where chemicals, stains, microbiological cultures, and glassware or microscope slides are used:
1. A full length lab coat that is fully buttoned to avoid contamination of clothing and skin.
2. OSHA recommended safety goggles ANSI Z87.1 splash goggles. These will be provided in lab.
3. Gloves. These will be provided in lab.
4. At the end of lab, gloves must be removed safely and disposed of only in the designated biohazard containers. Do NOT dispose of gloves in the trash can!
Safe removal of gloves:
a. Pinch the palm of the first glove and remove by pulling the glove inside out as shown below. (Fig. 1A)
Fig. 1A: Removing First Glove
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
b. Hold the first glove with your remaining gloved hand, slide your fingers inside the second glove and turn it inside out as it is removed. (Fig. 1B)
Fig. 1B: Removing Second Glove
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
c. Dispose of the gloves in the correct biohazard container, NOT in the trash can.
1. Anyone working with bacterial cultures, chemicals, and/or projectile and sharps hazards must wear:
a. Approved (ANSI Z87.1) safety goggles;
b. Gloves; and
c. A lab coat buttoned all the way to the bottom.Goggles and gloves will be provided in the lab but you must purchase your own lab coat at the college bookstore. Lab coats cannot be removed from a microbiology lab. They will be stored in the lab and must be discarded at the end of the semester.
2. Always familiarize yourself in advance with the exercises to be performed.3. Disinfect the bench tops with Lysol before and after each lab.
4. The first part of each lab period will be used to complete and record the results of prior experiments. When you come into the lab, always pull out and organize any culture tubes or petri plates you have in the incubator from previous labs. We will always go over these results as a class. You may wish to purchase a set of colored pencils to aid you in recording your results in the lab manual.
5. The latter part of each lab period will be used to begin new experiments. Preliminary instructions, demonstrations, and any changes in procedure will be given by your instructor prior to starting each new lab exercise.
6. After completing an experiment, dispose of all laboratory media and contaminated materials in the designated areas as described above.
7. Sanitize your hands or wash them with soap and water for 20 seconds before leaving the lab.
Return to Menu for Introduction
E. Binomial Nomenclature
Microorganisms are given specific scientific names based on the binomial (two names) system of nomenclature. The first name is referred to as the genus and the second name is termed the species. The names usually come from Latin or Greek and describe some characteristic of the organism.
To correctly write the scientific name of a microorganism, the first letter of the genus should be capitalized while the species name should be in lower case letters. Both the genus and species names are italicized or underlined. Several examples are given below.
Bacillus subtilus
Bacillus: L. dim. noun Bacillum, a small rod
subtilus: L. adj. subtilus, slenderEscherichia coli
Escherichia: after discoverer, Prof. Escherich
coli: L. gen. noun coli, of the colonStaphylococcus aureus
Staphylococcus: Gr. noun Staphyle, a bunch of grapes; Gr. noun coccus, berry
aureus: L. adj. aureus, golden
Return to Menu for Introduction
F. Metric Length and Fluid Volume
The study of microorganisms necessitates an understanding of the metric system of length. The basic unit of length is the meter (m), which is approximately 39.37 inches. The basic unit for fluid volume is the liter (l), which is approximately 1.06 quarts. The prefix placed in front of the basic unit indicates a certain fraction or multiple of that unit. The most common prefixes we will be using are:
-
centi (c) = 10-2 or 1/100
centimeter (cm) = 10-2 m or 1/100 m
- milli (m) = 10-3
or 1/1000
millimeter (mm) = 10-3 m or 1/1000 m
milliliter (ml) = 10-3 l or 1/1000 l - micro (µ)= 10-6 or 1/1,000,000
- nano (n)= 10-9 or 1/1,000,000,000
micrometer (µm) = 10-6 m or 1/1,000,000 m
microliter (µl) = 10-6 l or 1/1,000,000 l
nanometer (nm) = 10-9 m or 1/1,000,000,000 m
In microbiology, we deal with extremely small units of metric length (micrometer, nanometer). The main unit of length is the micrometer (µm) which is 10-6 (1/1,000,000) of a meter or approximately 1/25,400 of an inch.
The average size of a bacillus-shaped (cylindrical) bacterium (Fig. 2) is 0.5-1.0 µm wide by 1.0-4.0 µm long. An average coccus-shaped (spherical) bacterium (Fig. 3) is about 0.5-1.0 µm in diameter. A volume of one cubic inch is sufficient to contain approximately nine trillion average-sized bacteria. It would take over 18,000,000 average-sized cocci lined up edge-to-edge to span the diameter of a dime!
In several labs we will be using
pipettes to measure fluid volume in ml.
Return to
Menu for Introduction G. Using
the Microscope (Olympus Model CX31 Microscope)


Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.

Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
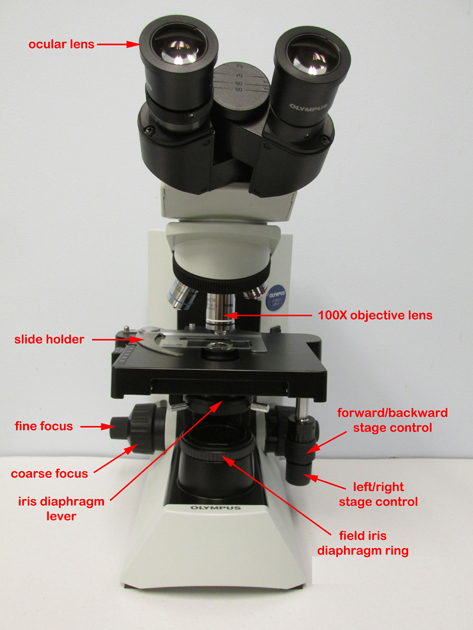
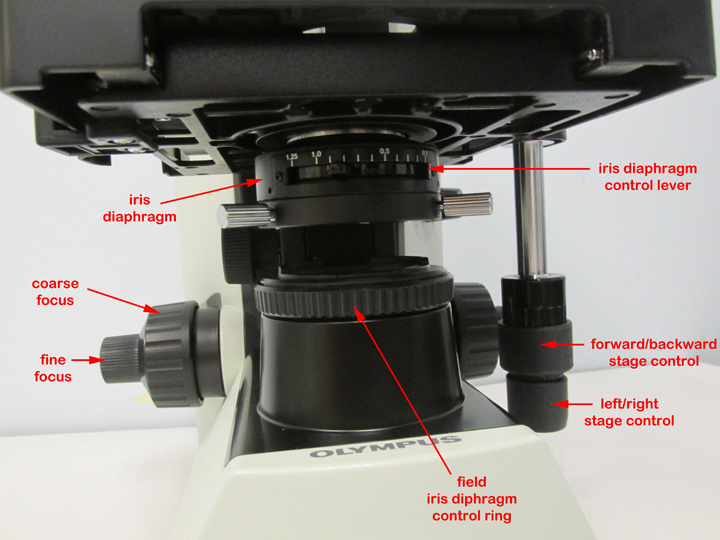
1. Moving and transporting the microscope
Grasp the arm of the microscope with one hand and support the base of the microscope with the other. Handle the microscope gently, it costs over $2000.
2. Before you plug in the microscope, turn the light intensity control dial (see Fig. 4) on the right side of the microscope to 1. Now plug in the microscope and use the on/off switch on the right side of the microscope to turn it on (see Fig. 4)). Make sure the entire cord is on the bench top and not hanging down where it could be caught by a leg. Adjust the voltage control dial to 6.
Fig. 4: Olympus CX31 Microscope
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
3. Adjusting the eyepieces (see Fig. 5)
These microscopes are binocular, that is, they have 2 ocular lenses (eyepieces). To adjust them, first find the proper distance between your eyes and the eyepieces by closing one eye and slowly moving your head toward that eyepiece until you see the complete field of view - about 1 inch away. Keep your head steady and both eyes in the same plane. Now open the other eye and gradually increase the other eyepiece up or down until it matches the distance between your eyes. At the correct distance you will see one circular field of view with both eyes.
Fig. 5: Olympus CX31 Microscope
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
4. Positioning the slide
Place the slide specimen-side-up on the stage so that the specimen lies over the opening for the light in the middle of the stage. Secure the slide between - not under- the slide holder arms of the mechanical stage (see Fig. 6). The slide can now be moved from place to place using the 2 control knobs located under the stage on the right of the microscope (see Fig. 4).
Fig. 6: Olympus CX31 Microscope Slide Holder
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
5. Adjusting the illumination
a. Adjust the light intensity by turning the light intensity control dial located on the rear right-hand side of the microscope (see Fig. 4). For oil immersion microscopy (1000X) set the light on 6. At lower magnifications less light may be needed.
b. Adjust the amount of light coming through the condenser using the iris diaphragm lever located under the stage in the front of the microscope (see Fig. 7). Light adjustment using the iris diaphragm lever is critical to obtaining proper contrast. For oil immersion microscopy (1000X), the iris diaphragm lever should be set almost all the way open (to your left for maximum light). For low powers such as 100X the iris diaphragm lever should be set mostly closed (to your right for minimum light).
Fig. 7: Iris Diaphragm
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
c. The condenser height control (the single knob under the stage on the left-hand side of the microscope should be set so the condenser is all the way up.
6. Obtaining different magnifications
The final magnification is a product of the 2 lenses being used. The eyepiece or ocular lens magnifies 10X. The objective lenses (see Fig. 5) are mounted on a turret near the stage. The small yellow-striped lens magnifies 10X; the blue-striped lens magnifies 40X, and the white-striped oil immersion lens magnifies 100X. Final magnifications are as follows:
total magnification 100X 400X 1000X 7. Focusing from lower power to higher power
Video lesson - Focusing Using Low Magnifications: Using the 10X Objective
a. Rotate the yellow-striped 10X objective until it locks into place (total magnification of 100X).
b. Turn the coarse focus control (larger knob; Fig. 5) all the way away from you until it stops.
c. Look through the eyepieces and turn the coarse focus control (larger knob) towards you slowly until the specimen comes into focus.
d. Get the specimen into sharp focus using the fine focus control (smaller knob; see Fig. 4) and adjust the light for optimum contrast using the iris diaphragm lever.
e. If higher magnification is desired, simply rotate the blue-striped 40X objective into place (total magnification of 400X) and the specimen should still be in focus. (Minor adjustments in fine focus and light contrast may be needed.)
f. For maximum magnification (1000X or oil immersion), rotate the blue-striped 40X objective slightly out of position and place a drop of immersion oil on the slide. Now rotate the white-striped 100X oil immersion objective into place. Again, the specimen should remain in focus, although minor adjustments in fine focus and light contrast may be needed.
Directions for focusing directly with oil immersion (1000X) without first focusing using lower powers will be given in Laboratory 1.
Video lesson - Focusing Using Oil Immersion (1000X) Microscopy
8. Cleaning the microscope
Clean the exterior lenses of the eyepiece and objective before and after each lab using lens paper only. (Paper towel or Kim-wipes may scratch the lens.)Remove any immersion oil from the oil immersion lens before putting the microscope away.
9. Reason for using immersion oil
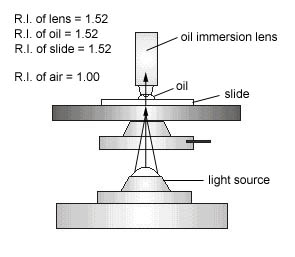
Normally, when light waves travel from one medium into another, they bend (see Fig. 8). Therefore, as the light travels from the glass slide to the air, the light waves bend and are scattered similar to the "bent pencil" effect when a pencil is placed in a glass of water. The microscope magnifies this distortion effect. Also, if high magnification is to be used, more light is needed.
Immersion oil has the same refractive index as glass (see Fig. 9A and Fig. 9B) and, therefore, provides an optically homogeneous path between the slide and the lens of the objective. Light waves thus travel from the glass slide, into glass-like oil, into the glass lens without being scattered or distorting the image (see Fig. 10). In other words, the immersion oil "traps" the light and prevents the distortion effect that is seen as a result of the bending of the light waves.
Fig. 8: Image Distortion as a Result of Light Moving from Air into Water
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Fig. 9A: The Refractive Index of Immersion Oil: Dropper Filled with Air
Fig. 9B: The Refractive Index of Immersion Oil: Dropper Filled with Oil
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Fig. 10: Using Immersion Oil to Create an Optically Homogeneous Light Path
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Return to Menu for Introduction
PERFORMANCE OBJECTIVES
FOR THE INTRODUCTION
After completing this introduction, the student will be able to perform the following objectives:
A. USING PERFORMANCE OBJECTIVES
1. Answer all performance objectives as soon as possible after completing each laboratory exercise.
B. LABORATORY RULES
1. Follow all laboratory rules stated in the Introduction.
C. GENERAL DIRECTIONS
1. Follow all general directions stated in the Introduction.
D. BINOMIAL NOMENCLATURE
1. Define genus and species and state how to correctly write the scientific name of a microorganism.
2. Be able to correctly write the scientific names of microorganisms.
E. METRIC LENGTH
1. Define and give the commonly-used abbreviations for the following units of metric length and fluid volume: centimeter, millimeter, micrometer, nanometer, milliliter, and microliter.
2. State the length and width of an average bacillus-shaped bacterium and the diameter of an average coccus-shaped bacterium in micrometers.
F. USING THE MICROSCOPE
1. Correctly clean the eyepiece and the objective lenses before and after each lab.
2. Define ocular lens and objective lens.
3. Place a slide in the slide holder of a mechanical stage correctly.
4. Focus on a specimen using 10X, 40X, and 100X objectives.
5. Adjust the light using the iris diaphragm lever for optimum contrast after focusing.
6. State the reason for using immersion oil at 1000X.
7. Calculate the total magnification of a lens system when using a 10X, 40X, or 100X objective in conjunction with a 10X eyepiece.
Return to Menu for Introduction
SELF-QUIZ
Return to Menu for Introduction

Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Last updated: February, 2023
Please send comments and inquiries to Dr.
Gary Kaiser